Case 2 170519-2 (S1700153-3)
Conference Coordinator: Charles Alex
//
One-year-old male red-flanked duiker (Cephalophus rufilatus).
Limited clinical history is available. The patient was anorectic and dull, and died.
The patient was an 8.8 kg red-flanked duiker in thin body condition, with scant fat. Ocular and gingival mucosae were red. Palpable lymph nodes were slightly enlarged and red. The trachea and bronchi contained moderate amounts of white to yellow stable foam. The visceral pleura had a few fibrin strands. The lungs had numerous, 0.3- to 0.5-cm-diameter, coalescing red areas, which sometimes contained a white spot in the middle. The right apical lobe was red and firm. Mediastinal lymph nodes are moderately enlarged and red. The pericardial sac contained 5 ml of yellow fluid. The abdomen contained approximately 20 ml of yellow fluid, which was partially clotted, plus a few fibrin strands. The perirenal tissue had multifocal hemorrhages. The liver was markedly enlarged, with rounded margins and numerous pin-point foci dispersed throughout the parenchyma. The spleen was mildly enlarged. The kidneys were enlarged and had numerous irregular white areas along the surface. The cut surface displayed prominent cortical radiations and an orange color of the medulla. The urinary bladder mucosa was diffusely red with numerous petechial hemorrhages. Urinalysis provided the following values: protein (+++), pH 7.5, blood (++++), ketones (-), bilirubin (++) and glucose (-).
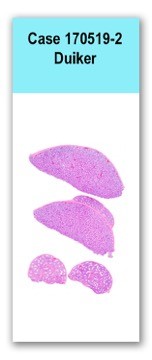
No significant findings were identified in the gastrointestinal tract. Mesenteric lymph nodes were moderately enlarged and red.The brain was slightly swollen, with mild coning of the cerebellum.This slide includes four sections of testicle and epididymis in which the interstitium is markedly expanded by a dense cellular infiltrate of mixed cells dominated by round cells resembling lymphoblasts, that pack and separate seminiferous tubules and efferent epidydimal ducts. The lymphoblast-like cells have fairly distinct cell borders, scant to small amounts of, pale-eosinophilic, slightly granular cytoplasm, and a single round to ovoid, central nucleus with coarsely-clumped chromatin and indistinct nucleolus. Anisocytosis in this population is mild, anisokaryosis is mild to moderate, and there are three mitotic figures in ten 400x fields. Moderate numbers of small lymphocytes and fewer macrophages are admixed with this population. Small- to medium-sized vessels are partially filled by the same cell population. Scattered plasma cells and macrophages are admixed with the blastic cell population. Approximately 80% of the seminiferous tubules and efferent ducts are at various stages of degeneration with moderate to marked reduction in spermatogenesis. Affected seminiferous tubules are characterized by marked depletion of mature spermatids, reduction germinal cells and Sertoli cells, and frequent proliferation of immature multinucleated spermatids admixed with individual apoptotic and necrotic cells within the tubular lumina. Degenerate efferent ductules are moderately to markedly depleted of spermatozoa and lined by fragmented/necrotic columnar principal cells and a variably thickened and undulating basal cell layer.
Polymerase chain reaction (PCR) assays for known gammaherpesviruses in the malignant catarrhal fever group were negative.
A PCR using degenerate primers amplified a 1kb region of a novel viral genome (see comment). Aerobic cultures of liver, lung, and urine were negative. Aerobic cultures of kidney and mesenteric lymph nodes grew small numbers of Streptococcus lutetiensis.A fluorescent antibody assay for Leptospira was performed on a kidney impression smear, but it was negative.
A PCR for bovine leucosis virus was negative.
PCR for Mycobacterium avium ssp. paratuberculosis was negative.
Salmonella PCR and cultures (liver, mesenteric lymph node, small intestine) were negative.
Fecal flotation did not demonstrate parasite eggs.
A heavy metal screen was interpreted as within normal limits, although reference ranges for this species were not available.
Immunohistochemistry on a section of kidney demonstrated CD3+ immunoreactivity in approximately 80% of the proliferative round cells. Admixed CD79a+ cells comprised 10-20% of the round cells. They were negative for CD204.
Virus isolation from pooled tissues and electron microscopy of cell cultures did not identify any viruses.
Testis and epididymis: Marked interstitial lymphoproliferative infiltration
Thyroid carcinomas occur more commonly in dogs (90%) than thyroid adenomas (9%). There is no sex predilection for thyroid carcinomas in dogs, and Boxers, beagles, Siberian huskies, and golden retrievers have a higher incidence compared to other breeds (1). Unlike cats, most dogs with functional thyroid neoplasms are euthyroid or have mildly elevated T4/T3 values. Clinical signs in dogs may include polyuria, polydipsia, weight loss despite increased appetite, muscle weakness, heat intolerance, and nervousness. Large, invasive tumors may cause dysphagia or dyspnea (1).
Histologically, thyroid carcinomas are classified as follicular, solid/compact, or papillary based on the predominant pattern of growth (1). In follicular thyroid carcinomas, neoplastic cells form follicular structures that often contain clumped and mineralized colloid (1). Solid or compact thyroid carcinomas form solid sheets of neoplastic cells separated by fibrous stroma, and immunohistochemistry for thyroglobulin, thyroid transcription factor 1, or calcitonin may be necessary to distinguish these tumors from C-cell carcinomas (1). Follicular to solid type carcinomas are the most common subtype in dogs. Papillary thyroid carcinomas form papillary projections that extend into cystic spaces within the mass, and are rare in domestic species compared to humans (1). Undifferentiated thyroid carcinomas, including small cell and giant cell variants, are uncommon in domestic animals (1). Canine thyroid carcinomas are often partially encapsulated and contain large central areas of necrosis and hemorrhage. Areas of mineralization or bone formation can also occur within the mass (1). Osseous and chondroid metaplasia of the stroma has been reported in canine thyroid carcinomas (2, 3). Malignant mixed thyroid tumors can also occur, in which both the follicular cells and mesenchymal components of the mass are malignant. The mesenchymal components are often osteogenic or cartilaginous, and may appear similar to osteosarcoma or chondrosarcoma (1, 4). Thyroid carcinomas eventually breach their capsule and invade into local structures including the trachea, esophagus, and larynx. Invasion into the thyroid or jugular veins creates neoplastic thrombi, which often leads to metastasis to the lungs (1).1. Meuten DJ, editor. Tumors of domestic animals, 5th edition. Ames, IA: John Wiley & Sons, Inc.; 2017. p. 791-799.
2. Kobayashi R, Yamada N, Kitamori T, Kitamori F, Sato K, Doi T, Wako Y, Sato J, Tsuchitani M. Follicular thyroid carcinoma characterized by adundant stromal components with chondroid and osseous metaplasia in a dog. Journal of Veterinary Medical Science. 2014. 76(8): 1161-1164. 3. Brodey RS, Kelly DF. Thyroid neoplasms in the dog: a clinicopathologic study of fifty-seven cases. Cancer. 1968. 22(2): 406-416. 4. Grubor B, Haynes JS. Thyroid carcinosarcoma in a dog. Veterinary Pathology. 2005. 42: 84-87.